Back to blog
Accelerate Medical-Device Regulatory Audits
Boston, United States
Project Overview
Strategy & Advisory
AI Products & Platforms
Knowledge Systems
Automation & Integration
AI Infrastructure
Governance & Risk
A specialized regulatory consulting firm serving medical-device manufacturers faced increasing complexity in audit preparation as global regulations evolved. Each audit required manual comparison of hundreds of regulatory clauses against dozens of internal SOPs, creating long lead times, high expert dependency, and elevated compliance risk.
SFAI Labs partnered with the firm to design an AI Product Strategy that transformed regulatory auditing into a scalable, technology-enabled workflow. The engagement focused on building a secure, private AI system capable of mapping global regulations to internal quality-system elements while preserving strict data governance.
Within months, SFAI Labs delivered a production-ready regulatory intelligence platform integrating structured ingestion, domain-specific RAG, multi-stage compliance evaluation, and human-in-the-loop verification. The system enabled consistent, explainable, and auditable regulatory assessments across jurisdictions and product types.
The result was a differentiated AI-powered audit assistant that reduces preparation time, improves audit accuracy, and creates a foundation for long-term product commercialization.
Key Takeaways
Domain knowledge drives accuracy
Evidence-based AI builds trust
Governance enables adoption
Evaluation prevents false compliance
Strategy enables monetization
Challenge
Medical-device regulations such as FDA QSR, ISO 13485, and EU MDR contain thousands of evolving requirements mapped across more than 30 quality-system elements. Experts manually reviewed these regulations against large libraries of SOPs, often taking weeks per audit. Terminology inconsistencies, cross-references, and undocumented dependencies created hidden gaps that were difficult to detect. Data sensitivity further constrained the use of public AI tools.
Strategy
SFAI Labs defined a strategy centered on regulatory-grade accuracy, privacy-by-design, and explainability. We designed a multi-layer system combining curated regulatory databases, controlled vocabularies, RAG pipelines, and confidence-based evaluation. The roadmap prioritized high-risk compliance areas first, followed by cross-regulation intelligence and automated gap detection.
Solution
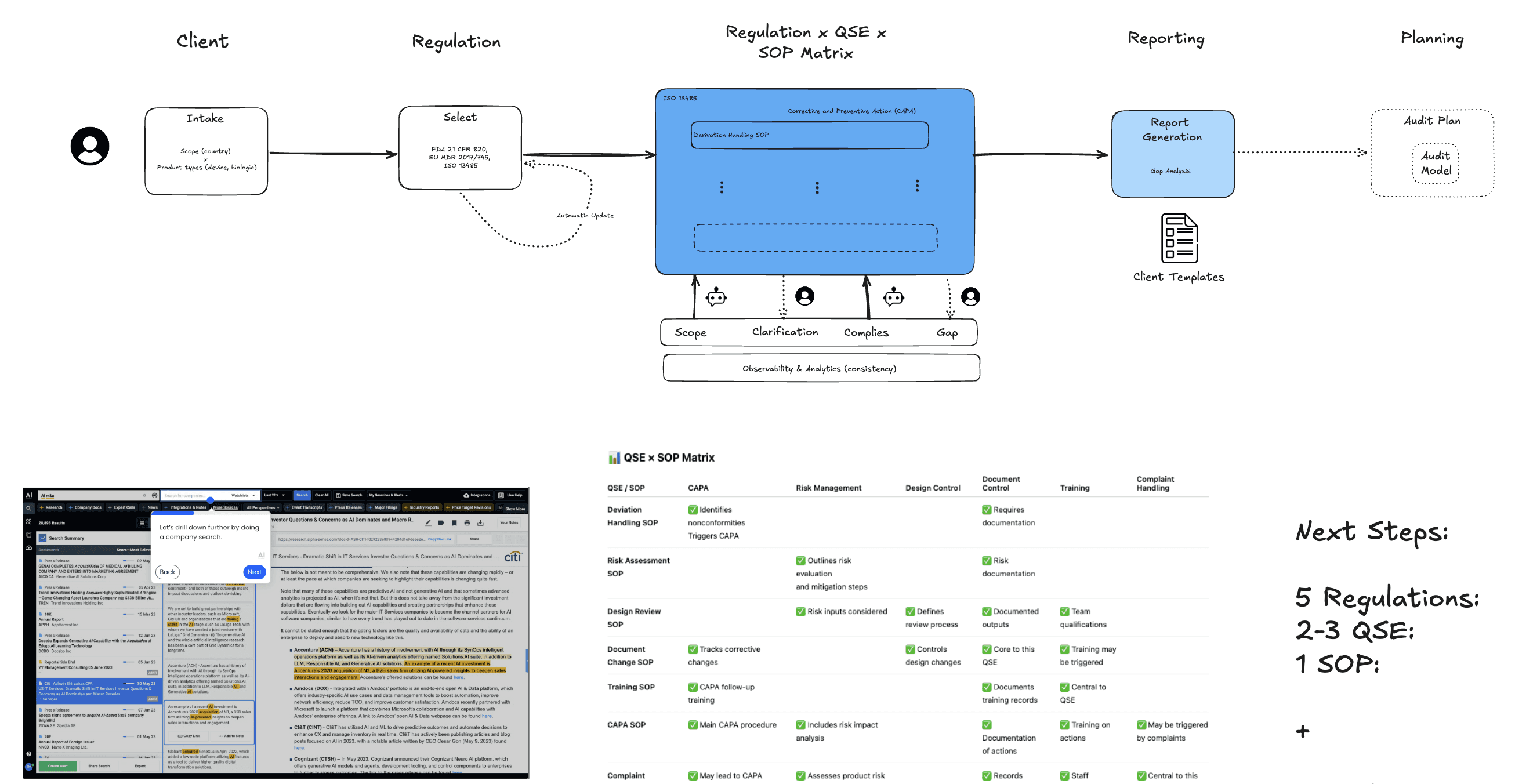
We designed an AI-driven regulatory audit platform featuring:
Version-controlled regulatory knowledge base
Secure, tenant-isolated RAG engine
Two-stage compliance evaluation framework
Evidence citation and quotation engine
Confidence scoring and anomaly detection
Human-in-the-loop review interface
The system enabled auditors to inspect, validate, and export structured audit reports with full traceability.
Execution
Phase 1: Regulatory ingestion and schema design
Phase 2: RAG and terminology alignment engine
Phase 3: Compliance classification and evidence extraction
Phase 4: Evaluation metrics and anti-hallucination controls
Phase 5: Backend deployment and security hardening
Phase 6: Alpha release and client validation
Results
Audit preparation time reduced by 60–80%
High-confidence regulation matching achieved across major domains
Production-ready ISO 13485 audit workflow delivered
Business Value
The engagement enabled the firm to scale regulatory audits without proportional increases in senior regulatory staff. Automation improved delivery predictability, strengthened audit defensibility, and positioned the firm to serve clients without enterprise eQMS systems. The platform also created new recurring revenue opportunities through subscription-based compliance intelligence.
Why SFAI Labs
SFAI Labs combined regulatory-domain expertise with advanced AI system design and enterprise governance. Our lab acceleration model enabled rapid prototyping, rigorous evaluation, and secure deployment—ensuring the solution met both commercial growth objectives and regulatory-grade reliability.





